Intermolecular Forces Unit Bundle Guided Notes
Kelsey Chemistry
505 Followers
Grade Levels
9th - 12th
Subjects
Resource Type
Standards
NGSSHS-PS1-3
Formats Included
- Zip
Pages
5 days guided notes & slides, 3 versions unit test, 4 vocab resources + matching quiz, 5 worksheets
Kelsey Chemistry
505 Followers
What educators are saying
Great resource for me (a first year chemistry teacher) to teach IMFs in a way that makes sense to myself and my students.
Products in this Bundle (6)
showing 1-5 of 6 products
Bonus
Unit Plan & Full Unit Slide Decks
Also included in
- A full year chemistry curriculum that contains 98 guided notes lessons for your students with 4 final exams to finish your school year. This bundle is growing and will soon include vocabulary activities and quizzes, and unit tests for every unit. Download the preview file to get a look and the breakPrice $335.00Original Price $482.50Save $147.50
Description
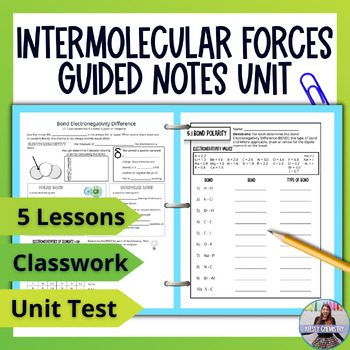
My collection of intermolecular forces lessons. Students will learn polar and nonpolar bonds and molecules. Then the three types of intermolecular forces: dipole-dipole, hydrogen bonding and dispersion forces. Finally learning the Effects of Intermolecular Forces
- Bond Polarity
- Molecule Polarity
- Dipole-dipole Interactions and Hydrogen-Bonding
- Dispersion Forces
- Effects of Intermolecular Forces
FEATURES
- Guided notes that have students listening to you instead of writing notes.
- Accurate diagrams, so students don't have to rely on their art skills to learn.
- Two formats of notes - full page and half page - perfect for packets, binders or interactive notebooks.
WHAT'S INCLUDED?
- 6 Slide Decks (x3 verisons: Whimsical, Classical and Dark Mode)
- A 15-ish minute lesson with guided notes in 2 styles: full page or half page (for interactive notebooks)
- Links to Copy Google Slides
- Show What You Know Questions designed for Self Assessment for each lesson
- 5 quick practice worksheets, 1 per lesson for a short classwork assignment
- 5 Vocabulary resources: a list of terms, 1 set of flash cards, a joke decipher puzzle, a crossword, Term-Definition Dominoes and 3 versions of a matching quiz
- 5 lesson plans in the 5Es style
- Full All Star Planning Style Unit Plan
- List of learning targets and vocab terms to act as a student study guide
- 3 versions of a 25 question unit test: 20 MC questions and 5 written questions
NOT SURE WHERE THIS FITS IN YOUR SCHOOL YEAR?
Check out my free chemistry curriculum outline for my suggestions!
ALSO INCLUDED IN:
This product by Kelsey Reavy is copyrighted for single classroom only. This product may not be resold and can be copied for personal use within a classroom only. If you have questions, please email kelsey@kelseyreavy.com © Kelsey Reavy
Total Pages
5 days guided notes & slides, 3 versions unit test, 4 vocab resources + matching quiz, 5 worksheets
Answer Key
Included
Teaching Duration
1 Week
Report this resource to TPT
Reported resources will be reviewed by our team. Report this resource to let us know if this resource violates TPT’s content guidelines.
Standards
to see state-specific standards (only available in the US).
NGSSHS-PS1-3
Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles. Emphasis is on understanding the strengths of forces between particles, not on naming specific intermolecular forces (such as dipole-dipole). Examples of particles could include ions, atoms, molecules, and networked materials (such as graphite). Examples of bulk properties of substances could include the melting point and boiling point, vapor pressure, and surface tension. Assessment does not include Raoult’s law calculations of vapor pressure.