Atomic Structure Bohr Diagrams for the First 20 Elements Distance Learning
- PDF
What educators are saying
Also included in
- Are you tired of boring, run-of-the-mill teaching resources that make you want to hide under your lab coat? Well, fear not, because here is the ultimate, out-of-your-element bundle for atomic structure and patterns in the periodic table that will have your students shouting "Eureka!" in no time!It bPrice $47.70Original Price $53.00Save $5.30
- Simple and effective graphical organizers for electron configuration. Original artwork.For more detail, please check out each product individually.Click here to follow my store and receive the latest updates.Original Artwork (©AwesomeScience). For Personal Use Only. Uneditable.Page count does not inPrice $5.40Original Price $6.00Save $0.60
- The complete, no prep., bundle containing:Bohr-Rutherford Diagrams for the 1st Twenty Elements, with Answers;Lewis Dot / Valence Electron Diagrams for the 1st Twenty Elements with Answers;&Bohr models for all elements on the periodic table (1-118).Click here to follow my store and receive the laPrice $9.00Original Price $10.00Save $1.00
Description
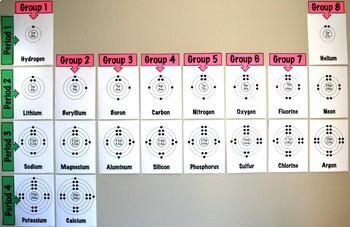
Easily compare and contrast the location and electron configuration of the first twenty elements with this handy graphic organizer. Great transition to patterns in the periodic table (periods and groups).
*UPDATED* now includes Bohr model posters (8 ½ x 11 inches) for the first twenty elements. Create a large “Patterns in the Periodic Table” chart on the wall of your classroom!
Supplementary resources available here:
- Free - Bohr Rutherford Diagrams for the 1st Twenty Elements Graphic Organizer
- Bohr-Rutherford Diagrams for the 1st Twenty Elements - with Answers
- Free - Lewis Dot / Valence Electrons for the 1st Twenty Elements Graphic Organizer
- Lewis Structures Valence Electron Diagrams for 1st 20 Elements - With Answers
⭐Click here to follow my store & receive the latest updates.
Original Artwork (©AwesomeScience). For Personal Use Only. Uneditable.
Page count does not include Terms of Use and links to supplementary activities.
Copyright ©AwesomeScience 2013 – The Present.
All Rights Reserved by Author.
By using this Resource you agree to the Terms as outlined in the Terms of Service. This Resource is for limited Personal Use only; not to be used, in part or in whole, for commercial purposes. Each Individual License is for use by one specific educator only. Additional licenses must be purchased for each additional educator. Except as permitted in Section 3 to deliver Resources electronically to Permitted Recipients, you may not post or otherwise make the Resource available on any website, application, shared drive or other sites or services.