Chemistry PowerPoint: Chemical Reactions
- PPTX
- Prezis
Also included in
- Make teaching easier with this High School Chemistry Growing Bundle of resources. Unit plans 1-7 are already included as well as labs, worksheets, and virtual activities. This resource will be updated periodically!I have created this bundle with some of my favorite resources. I hope you find themPrice $135.06Original Price $187.58Save $52.52
Description
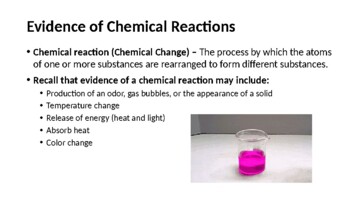
This comprehensive PowerPoint is ideal for secondary chemistry, physical science, honors, or general science students and is fully modifiable for any grade level. Consisting of 24 detailed slides, this PowerPoint was created to help make teaching easier and save you time. Use this resource to help students learn how to identify and balance the following types of chemical equations: Synthesis, Decomposition, Single Replacement, Double Replacement, and Combustion. Students will learn about indicators as evidence of a chemical reaction (specifically formation of precipitates, gas production, water production, and changes in energy to the system).
Content objectives covered in this unit plan include (TSWBAT):
- Recognize evidence of chemical change
- Represent chemical reactions with equations.
- Classify chemical reactions.
- Identify the characteristics of different classes of chemical reactions.
- Describe aqueous solutions.
- Predict whether reactions in aqueous solution swill produce precipitate, water, or a gas.
Language Objectives: SWBAT
- Read, define, and discuss key vocabulary terms: chemical reaction, reactant, product, chemical equation, coefficient, synthesis reaction, combustion reaction, decomposition reaction, single-replacement reaction, double-replacement reaction, precipitate reaction, and precipitate.
- Listen and write key points discussed in lecture.
- Write complete ionic equations for chemical reactions in aqueous solutions.
- Analyze and interpret conclusions based on experimental data gathered from accurate measurement procedures.
Key Vocabulary: chemical reaction, reactant, product, chemical equation, coefficient, synthesis reaction, combustion reaction, decomposition reaction, single-replacement reaction, double-replacement reaction, precipitate reaction, and precipitate
Also available:
Find other unit plans, unit bundles, and chemistry worksheets to make teaching easier here!
For other great resources to make teaching easier follow me for updates!
- If you like my product, don't forget to write a review! Thank you!
- Visit my blog at www.maketeachingeasy.com!
- Contact me at bestforteachers@gmail.com with questions or concerns! I will be happy to help you!
Visit my page for weight loss and fitness programs for educators by ReversAge Health & Fitness.