Acids and Bases Neutralization Reactions Worksheet
Fission Impossible
59 Followers
Grade Levels
9th - 12th
Subjects
Resource Type
Formats Included
- Word Document File
Pages
4 pages
Fission Impossible
59 Followers
What educators are saying
I thought this was a great way to reinforce the concept of neutralization reactions in the classroom. Practicing new concepts helps to retain the knowledge that the students learn in the classroom.
This resource was a perfect way to practice predicting products of an acid/base reaction. It was laid out in a simple, easy to understand format. I appreciated the included answer key.
Description
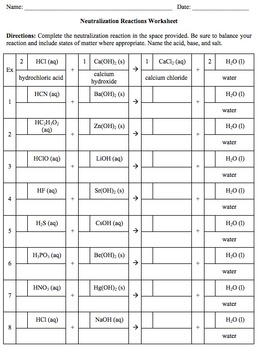
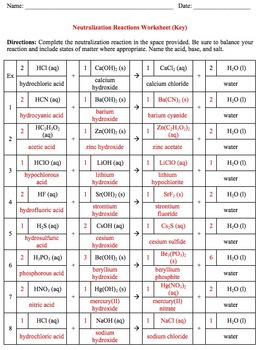
This document includes a neutralization reactions worksheet and the corresponding key. The worksheet requires each student to balance, identify the formula and phase of the salt formed, and name the acid, base, and salt in 18 neutralization reactions. The worksheet may take up to an entire class period depending on the skill level of your students.
Content Covered on Worksheet:
The worksheet covers 36 common acids and bases taught in general chemistry. Many cations were chosen because of their application when teaching solubility rules (i.e. lead, silver, mercury).
The worksheet covers the following 18 common acids - HF, HCl, HBr, HI, H2S, HNO3, HNO2, HClO, HClO2, HClO3, HClO4, H2SO4, H2SO3, H3PO4, H3PO3, H2CO3, HC2H3O2, and HCN.
The worksheet covers the following 18 common bases - LiOH, NaOH, KOH, RbOH, CsOH, Be(OH)2, Mg(OH)2, Ca(OH)2, Sr(OH)2, Ba(OH)2, Al(OH)3, Hg2(OH)2, Hg(OH)2, Zn(OH)2, AgOH, Pb(OH)4, Pb(OH)2, and NH4OH.
The worksheet covers the following solubility rules -
(1) All Group 1A, NH4+, NO3-, C2H3O2-, ClO3-, and ClO4- ions are soluble.
(2) Br-, Cl-, and I- are soluble except with Ag+, Hg2+2, Hg+2, Pb+2, and Pb+4.
(3) SO4-2 ions are soluble except with Ba+2, Sr+2, Ca+2, Ag+, Hg2+2, Hg+2, Pb+2, and Pb+4.
(4) S-2 ions are insoluble except with Group 1A, NH4+, Ca+2, Sr+2, and Mg+2.
(5) Except rule #1, consider all remaining ions to be insoluble.
Suggested Grading (See PREVIEW for Reference):
Because this is mainly a classroom assignment (and homework if necessary), I wouldn't grade every question for accuracy. I recommend grading questions 3, 6, 9, 12, 15, and 18 for accuracy. Each box would be worth 1 point and the entire box would have to be correct to receive credit. This would make each of these questions worth 8 points.
I would then grade the remaining questions for completion. That is, students would receive 4 points for a completely finished question, 2 points if not finished, and 0 points for no attempt.
This would make the entire worksheet worth a total of 96 points. Take how many points a student gets and divide it by the total points possible to get a percentage grade out of 100. (Example: Student Points Earned = 84, Student Points Possible = 96, Percentage Grade = 84/96 x 100 = 88%)
To Save Paper:
The worksheet is two pages in the document. Simply print out the document where the first part of the worksheet will be on the left-hand side of the paper and the second part of the worksheet will be on the right-hand side of the paper. This document contains 12-point Times New Roman Font which will remain easy for students to read once shrunk.
*This worksheet is fully customizable and any acids and bases you don't cover can be removed and replaced with those that you are.
Content Covered on Worksheet:
The worksheet covers 36 common acids and bases taught in general chemistry. Many cations were chosen because of their application when teaching solubility rules (i.e. lead, silver, mercury).
The worksheet covers the following 18 common acids - HF, HCl, HBr, HI, H2S, HNO3, HNO2, HClO, HClO2, HClO3, HClO4, H2SO4, H2SO3, H3PO4, H3PO3, H2CO3, HC2H3O2, and HCN.
The worksheet covers the following 18 common bases - LiOH, NaOH, KOH, RbOH, CsOH, Be(OH)2, Mg(OH)2, Ca(OH)2, Sr(OH)2, Ba(OH)2, Al(OH)3, Hg2(OH)2, Hg(OH)2, Zn(OH)2, AgOH, Pb(OH)4, Pb(OH)2, and NH4OH.
The worksheet covers the following solubility rules -
(1) All Group 1A, NH4+, NO3-, C2H3O2-, ClO3-, and ClO4- ions are soluble.
(2) Br-, Cl-, and I- are soluble except with Ag+, Hg2+2, Hg+2, Pb+2, and Pb+4.
(3) SO4-2 ions are soluble except with Ba+2, Sr+2, Ca+2, Ag+, Hg2+2, Hg+2, Pb+2, and Pb+4.
(4) S-2 ions are insoluble except with Group 1A, NH4+, Ca+2, Sr+2, and Mg+2.
(5) Except rule #1, consider all remaining ions to be insoluble.
Suggested Grading (See PREVIEW for Reference):
Because this is mainly a classroom assignment (and homework if necessary), I wouldn't grade every question for accuracy. I recommend grading questions 3, 6, 9, 12, 15, and 18 for accuracy. Each box would be worth 1 point and the entire box would have to be correct to receive credit. This would make each of these questions worth 8 points.
I would then grade the remaining questions for completion. That is, students would receive 4 points for a completely finished question, 2 points if not finished, and 0 points for no attempt.
This would make the entire worksheet worth a total of 96 points. Take how many points a student gets and divide it by the total points possible to get a percentage grade out of 100. (Example: Student Points Earned = 84, Student Points Possible = 96, Percentage Grade = 84/96 x 100 = 88%)
To Save Paper:
The worksheet is two pages in the document. Simply print out the document where the first part of the worksheet will be on the left-hand side of the paper and the second part of the worksheet will be on the right-hand side of the paper. This document contains 12-point Times New Roman Font which will remain easy for students to read once shrunk.
*This worksheet is fully customizable and any acids and bases you don't cover can be removed and replaced with those that you are.
Total Pages
4 pages
Answer Key
Included
Teaching Duration
N/A
Report this resource to TPT
Reported resources will be reviewed by our team. Report this resource to let us know if this resource violates TPT’s content guidelines.